
SL Paper 2
Define the term isotopes.
A sample of silicon contains three isotopes.
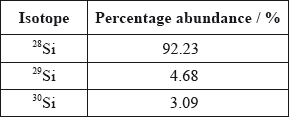
Calculate the relative atomic mass of silicon using this data.
Describe the structure and bonding in silicon dioxide and carbon dioxide.
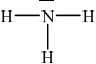
Draw the Lewis structure of NH3, state its shape and deduce and explain the H–N–H bond angle in \({\text{N}}{{\text{H}}_{\text{3}}}\).
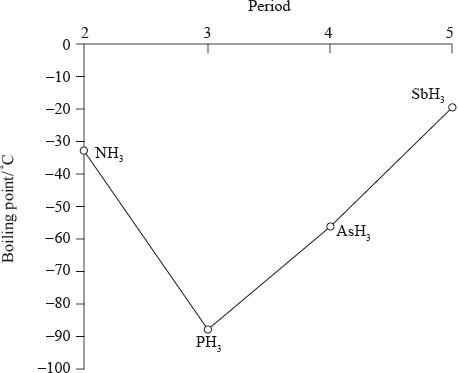
The graph below shows the boiling points of the hydrides of group 5. Discuss the variation in the boiling points.
Explain, using diagrams, why CO and \({\text{N}}{{\text{O}}_{\text{2}}}\) are polar molecules but \({\text{C}}{{\text{O}}_{\text{2}}}\) is a non-polar molecule.
Markscheme
atoms of the same element with the same atomic number/Z/same number of protons, but different mass numbers/A/different number of neutrons;
\((0.9223 \times 28) + (0.0468 \times 29) + (0.0309 \times 30)\);
28.1/28.11;
Working must be shown to get [2], do not accept 28.09 on its own (given in the data booklet).
Silicon dioxide
single covalent (bonds);
network/giant covalent/ macromolecular / repeating tetrahedral units;
Carbon dioxide
double covalent (bonds);
(simple / discrete) molecular;
Marks may be obtained from suitable structural representations of SiO2 and CO2.
 ;
;
Allow crosses or dots for lone-pair.
trigonal/triangular pyramidal;
(\( \sim \))107° / less than 109.5°;
Do not allow ECF.
LP-BP repulsion \( > \) BP-BP repulsion / one lone pair and three bond pairs / lone pairs/non-bonding pairs repel more than bonding-pairs;
Do not accept repulsion between atoms.
boiling points increase going down the group (from \({\text{P}}{{\text{H}}_{\text{3}}}\) to \({\text{As}}{{\text{H}}_{\text{3}}}\) to \({\text{Sb}}{{\text{H}}_{\text{3}}}\));
\({M_{\text{r}}}\)/number of electrons/molecular size increases down the group;
Accept electron cloud increases down the group for the second marking point.
greater dispersion/London/van der Waal’s forces;
\({\text{N}}{{\text{H}}_{\text{3}}}\)/ammonia has a higher boiling point than expected due to the hydrogen bonding between the molecules;
Do not accept hydrogen bonding alone.
CO:
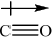
Award [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
\(N{O_2}\):
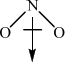
Award [1] for correct representation of the bent shape and [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
\(C{O_2}\):
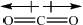
Award [1] for correct representation of the linear shape and [1] for showing the two equal but opposite dipoles or explaining it in words (symmetrical distribution of charge).
For all three molecules, allow either arrow or arrow with bar for representation of dipole moment.
Allow correct partial charges instead of the representation of the vector dipole moment.
Ignore incorrect bonds.
Lone pairs not needed.
Examiners report
In general the definition of isotopes was correct in (a) (i), but there are still some candidates who stated “isotopes are elements” and not “atoms of the same element”.
Nearly everybody gave the correct answer of 28.1 for the relative atomic mass of silicon in (ii).
Part (a) (iii) proved to be very difficult for the candidates. There was a lot of confusion about the two molecules; some candidates stated that they had the same double bond. Not many candidates mentioned the giant covalent structure for the silicon dioxide or the simple molecular structure for the carbon dioxide.
In (b) (i) the majority of candidates drew the Lewis structure of the ammonia molecule correctly showing the lone pair of electrons and the correct shape and angle and (ii) was well answered by most candidates.
They realised that \({\text{N}}{{\text{H}}_{\text{3}}}\) had a higher boiling point than \({\text{P}}{{\text{H}}_{\text{3}}}\) because of the intermolecular hydrogen bonding present in \({\text{N}}{{\text{H}}_{\text{3}}}\).
For (c) most answers given here showed diagrams of the three molecules, including distribution of charges, bonding and shapes. Some candidates gave very good answers showing a good understanding of the polarity of molecules.
If white anhydrous copper(II) sulfate powder is left in the atmosphere it slowly absorbs water vapour giving the blue pentahydrated solid.
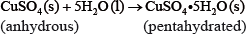
It is difficult to measure the enthalpy change for this reaction directly. However, it is possible to measure the heat changes directly when both anhydrous and pentahydrated copper(II) sulfate are separately dissolved in water, and then use an energy cycle to determine the required enthalpy change value, \(\Delta {H_{\text{x}}}\), indirectly.
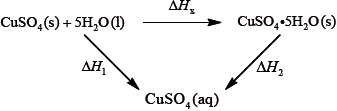
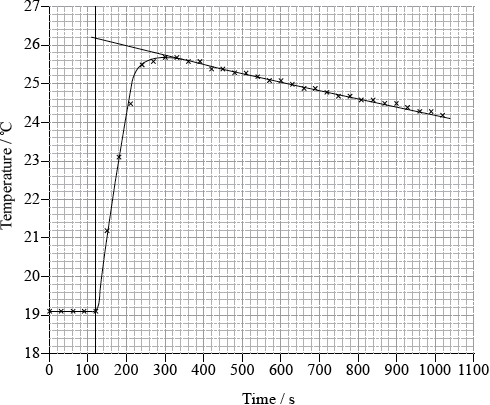
To determine \(\Delta {H_1}\) a student placed 50.0 g of water in a cup made of expanded polystyrene and used a data logger to measure the temperature. After two minutes she dissolved 3.99 g of anhydrous copper(II) sulfate in the water and continued to record the temperature while continuously stirring. She obtained the following results.
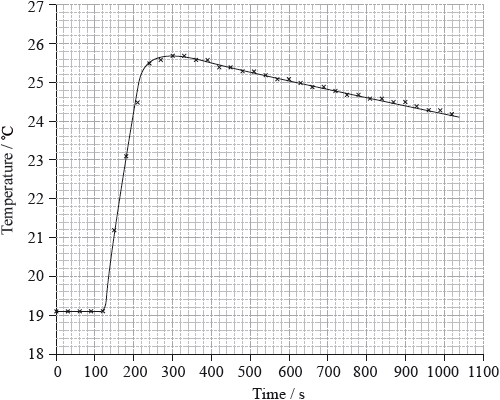
To determine \(\Delta {H_2}\), 6.24 g of pentahydrated copper(II) sulfate was dissolved in 47.75 g of water. It was observed that the temperature of the solution decreased by 1.10 °C.
The magnitude (the value without the \( + \) or \( - \) sign) found in a data book for \(\Delta {H_{\text{x}}}\) is \({\text{78.0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the amount, in mol, of anhydrous copper(II) sulfate dissolved in the 50.0 g of water.
Determine what the temperature rise would have been, in °C, if no heat had been lost to the surroundings.
Calculate the heat change, in kJ, when 3.99 g of anhydrous copper(II) sulfate is dissolved in the water.
Determine the value of \(\Delta {H_1}{\text{ in kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the amount, in mol, of water in 6.24 g of pentahydrated copper(II) sulfate.
Determine the value of \(\Delta {H_2}\) in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Using the values obtained for \(\Delta {H_1}\) in (a) (iv) and \(\Delta {H_2}\) in (b) (ii), determine the value for \(\Delta {H_{\text{x}}}\) in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Calculate the percentage error obtained in this experiment. (If you did not obtain an answer for the experimental value of \(\Delta {H_{\text{x}}}\) then use the value \({\text{70.0 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), but this is not the true value.)
The student recorded in her qualitative data that the anhydrous copper(II) sulfate she used was pale blue rather than completely white. Suggest a reason why it might have had this pale blue colour and deduce how this would have affected the value she obtained for \(\Delta {H_{\text{x}}}\).
Markscheme
\({\text{amount}} = \frac{{3.99}}{{159.61}} = 0.0250{\text{ (mol)}}\);
26.1 (°C);
Accept answers between 26.0 and 26.2 ( °C).
temperature rise \( = 26.1 - 19.1 = 7.0\) (°C);
Accept answers between 6.9 °C and (7.1 °C) .
Award [2] for the correct final answer.
No ECF if both initial and final temperatures incorrect.
heat change \( = \frac{{50.0}}{{1000}} \times 4.18 \times 7.0/50.0 \times 4.18 \times 7.0\);
Accept 53.99 instead of 50.0 for mass.
\( = 1.5{\text{ (kJ)}}\);
Allow 1.6 (kJ) if mass of 53.99 is used.
Ignore sign.
\(\Delta {H_1} = \frac{{1.5}}{{0.0250}} = - 60{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Value must be negative to award mark.
Accept answers in range –58.0 to –60.0.
Allow –63 (kJ\(\,\)mol–1) if 53.99 g is used in (iii).
\({\text{(amount of CuS}}{{\text{O}}_4} \bullet {\text{5}}{{\text{H}}_{\text{2}}}{\text{O}} = \frac{{6.24}}{{249.71}} = {\text{) 0.0250 (mol)}}\);
\({\text{(amount of }}{{\text{H}}_{\text{2}}}{\text{O in 0.0250 mol of CuS}}{{\text{O}}_{\text{4}}} \bullet {\text{5}}{{\text{H}}_{\text{2}}}{\text{O}} = 5 \times 0.0250 = {\text{) 0.125 (mol)}}\).
\((50.0 \times 4.18 \times 1.10 = ){\text{ }}230{\text{ (J)}}\);
\(\left( {\frac{{229.9}}{{(1000{\text{ }}0.0250)}} = } \right){\text{ }} + 9.20{\text{ (kJ)}}\);
Accept mass of 47.75 or 53.99 instead of 50.00 giving answers of +.8.78 or +9.9.
Do not penalize missing + sign but penalize – sign unless charge already penalized in (a) (iv).
\(\left( {\Delta {H_{\text{x}}} = \Delta {H_2} - \Delta {H_2} = - 58.4 - ( + 9.20) = } \right){\text{ }} - 67.6{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\)
\(\frac{{[ - 78.0 - ( - 67.6)]}}{{ - 78.0}} \times 100 = 13.3\% \);
If 70.0 kJ\(\,\)mol−1 is used accept 10.3%.
the anhydrous copper(II) sulfate had already absorbed some water from the air / OWTTE;
the value would be less exothermic/less negative than expected as the temperature increase would be lower / less heat will be evolved when the anhydrous salt is dissolved in water / OWTTE;
Do not accept less without a reason.
Examiners report
Question 1 was a generally difficult question for candidates, but most students did pick up marks thanks to the application of error carried forward (ecf). In part (a) students could usually calculate the moles of anhydrous copper sulphate.
Very few candidates could correctly extrapolate the graph to calculate a temperature rise of 7.0 ºC.
Calculating using \({\text{q}} = {\text{mc}}\Delta {\text{T}}\) also caused problems as many students used the mass of the copper sulphate instead of the mass of water, and some also added 273 to the temperature change. Many candidates also forgot to convert to kJ.
The last part of this question required the calculation of \(\Delta H\), here many students forgot the – symbol to indicate it was exothermic and so did not gain the mark.
In part (b) the problems were similar as students used incorrect values in their calculation but were able to obtain some marks by error carried forward.
In part (b) the problems were similar as students used incorrect values in their calculation but were able to obtain some marks by error carried forward.
In part (b) the problems were similar as students used incorrect values in their calculation but were able to obtain some marks by error carried forward.
In part (c) many could calculate the % error and apply Hess’s law to calculate \(\Delta H\). Throughout this question there were numerous instances of students using an incorrect number of significant figures and this led to another mark being lost.
Iron tablets are often prescribed to patients. The iron in the tablets is commonly present as iron(II) sulfate, \({\text{FeS}}{{\text{O}}_{\text{4}}}\).
Two students carried out an experiment to determine the percentage by mass of iron in a brand of tablets marketed in Cyprus.
Experimental Procedure:
• The students took five iron tablets and found that the total mass was 1.65 g.
• The five tablets were ground and dissolved in \({\text{100 c}}{{\text{m}}^{\text{3}}}\) dilute sulfuric acid, \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The solution and washings were transferred to a \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask and made up to the mark with deionized (distilled) water.
• \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of this \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was transferred using a pipette into a conical flask. Some dilute sulfuric acid was added.
• A titration was then carried out using a \(5.00 \times {10^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) standard solution of potassium permanganate, \({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The end-point of the titration was indicated by a slight pink colour.
The following results were recorded.
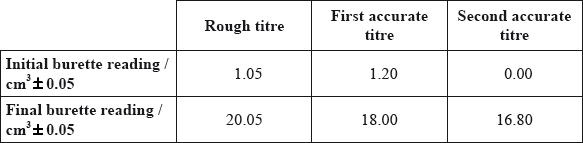
This experiment involves the following redox reaction.
\[{\text{5F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{MnO}}_4^ - {\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{5F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
When the \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was made up in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask, deionized (distilled) water was added until the bottom of its meniscus corresponded to the graduation mark on the flask. It was noticed that one of the two students measured the volume of the solution from the top of the meniscus instead of from the bottom. State the name of this type of error.
State what is meant by the term precision.
When the students recorded the burette readings, following the titration with KMnO4 (aq),the top of the meniscus was used and not the bottom. Suggest why the students read the top of the meniscus and not the bottom.
Define the term reduction in terms of electrons.
Deduce the oxidation number of manganese in the \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\) ion.
Determine the amount, in mol, of \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\), used in each accurate titre.
Calculate the amount, in mol, of \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) ions in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the solution.
Determine the total mass of iron, in g, in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) solution.
Determine the percentage by mass of iron in the tablets.
One titration was abandoned because a brown precipitate, manganese(IV) oxide, formed. State the chemical formula of this compound.
Markscheme
systematic (error);
Do not accept parallax.
closeness of agreement of a set of measurements to each other / OWTTE;
Allow reproducibility/consistency of measurement / measurements with small random errors/total amount of random errors/standard deviation / a more precise value contains more significant figures / OWTTE.
potassium permanganate has a very dark/deep (purple) colour so cannot read bottom of meniscus / OWTTE;
gain (of electrons);
VII / +7;
Do not accept 7 or 7+.
volume \( = 16.80{\text{ }}({\text{c}}{{\text{m}}^3})/18.00 - 1.20{\text{ }}({\text{c}}{{\text{m}}^3})\);
amount \(\left( { = \frac{{16.80 \times 5.00 \times {{10}^{ - 3}}}}{{1000}}} \right) = 8.40 \times {10^{ - 5}}{\text{ (mol)}}\);
Award [2] for correct final answer.
\((8.40 \times {10^{ - 5}} \times 5 \times 10) = 4.20 \times {10^{ - 3}}{\text{ (mol per 250 c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\((55.85 \times 4.20 \times {10^{ - 3}}) = 0.235{\text{ (g)}}\);
Do not penalize if 56 g mol–1 is used for atomic mass of iron.
\(\left( {\frac{{{\text{0.235}} \times {\text{100}}}}{{{\text{1.65}}}} = } \right){\text{ 14.2% }}\);
No ECF if answer >100 %.
MnO2;
Examiners report
Question 1 presented difficulties to many candidates. It is felt that the extended nature of the response distracted candidates from rather straight-forward quantitative chemistry calculations. Part (a) required candidates to determine whether an error was systematic or random and part (b) asked for the meaning of precision. Both of these questions are relevant to Topic 11.
Question 1 presented difficulties to many candidates. It is felt that the extended nature of the response distracted candidates from rather straight-forward quantitative chemistry calculations. Part (a) required candidates to determine whether an error was systematic or random and part (b) asked for the meaning of precision. Both of these questions are relevant to Topic 11.
Very few candidates related reading the top of the meniscus in the burette in part (c) to the colour of the KMnO4 solution. While it is acknowledged that few candidates would have performed this experiment themselves, it is reasonable that candidates should know the colour of KMnO4.
Part d) (i) was answered very well with nearly all candidates correctly defining reduction.
In d) (ii) many candidates correctly deduced the oxidation number of Mn in MnO4. Several lost marks, however, for not using acceptable notation. 7 by itself is not correct.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
Part (e) involved the calculations. Candidates were guided through the process of calculating number of moles from concentration and volume, finding mole ratios, and determining mass from moles and molar mass. Better candidates performed these calculations well. Weaker candidates often scored follow-through marks when working was shown.
In f) (i) a common error was to write Mn2O4 as the formula for manganese(IV) oxide. Also common was the use of the symbol Mg for manganese.
Ethanol is used as a component in fuel for some vehicles. One fuel mixture contains 10% by mass of ethanol in unleaded petrol (gasoline). This mixture is often referred to as Gasohol E10.
Assume that the other 90% by mass of Gasohol E10 is octane. 1.00 kg of this fuel mixture was burned.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(l)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{2C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 1367{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{C}}_8}{{\text{H}}_{18}}{\text{(l)}} + {\text{12}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{8C}}{{\text{O}}_2}{\text{(g)}} + {\text{9}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta {H^\Theta } = - 5470{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Calculate the mass, in g, of ethanol and octane in 1.00 kg of the fuel mixture.
Calculate the amount, in mol, of ethanol and octane in 1.00 kg of the fuel mixture.
Calculate the total amount of energy, in kJ, released when 1.00 kg of the fuel mixture is completely burned.
If the fuel blend was vaporized before combustion, predict whether the amount of energy released would be greater, less or the same. Explain your answer.
Markscheme
(10% 1000 g =) 100 g ethanol and (90% 1000 g =) 900 g octane;
\(n{\text{(ethanol)}} = 2.17{\text{ mol}}\) and \(n{\text{(octane)}} = 7.88{\text{ mol}}\);
\({{\text{E}}_{{\text{released from ethanol}}}} = (2.17 \times 1367) = 2966{\text{ (kJ)}}\);
\({{\text{E}}_{{\text{released from octane}}}} = (7.88 \times 5470) = 43104{\text{ (kJ)}}\);
total energy released \( = (2966 + 43104) = 4.61 \times {10^4}{\text{ (kJ)}}\);
Award [3] for correct final answer.
Accept answers using whole numbers for molar masses and rounding.
greater;
fewer intermolecular bonds/forces to break / vaporization is endothermic / gaseous fuel has greater enthalpy than liquid fuel / OWTTE;
M2 cannot be scored if M1 is incorrect.
Examiners report
Candidates were able to calculate the mass of ethanol and octane in the fuel mixture. The most common error here involved not expressing the answer in the requested units of grams. A number of candidates expressed answers in kg.
Many candidates were able to calculate the number of mole of ethanol and octane in (a) (ii) but errors in the calculation of molar mass were seen regularly. Candidates should also use the relative atomic masses, expressed to two decimal places as in the Periodic Table provided in the Data Table.
In part (a) (iii) some candidates multiplied incorrect numbers together or did not consider the number of moles of each part of the fuel mixture. Some candidates just added the enthalpies of combustion provided in the questions.
Part (b) was found to be very challenging by candidates. Very few candidates had the depth of understanding to answer this question adequately.
Aspirin, one of the most widely used drugs in the world, can be prepared according to the equation given below.
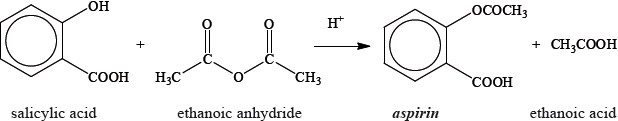
A student reacted some salicylic acid with excess ethanoic anhydride. Impure solid aspirin was obtained by filtering the reaction mixture. Pure aspirin was obtained by recrystallization. The following table shows the data recorded by the student.

State the names of the three organic functional groups in aspirin.
Determine the amount, in mol, of salicylic acid, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OH)COOH}}\), used.
Calculate the theoretical yield, in g, of aspirin, \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{\text{(OCOC}}{{\text{H}}_{\text{3}}}{\text{)COOH}}\).
Determine the percentage yield of pure aspirin.
State the number of significant figures associated with the mass of pure aspirin obtained, and calculate the percentage uncertainty associated with this mass.
Another student repeated the experiment and obtained an experimental yield of 150%. The teacher checked the calculations and found no errors. Comment on the result.
The following is a three-dimensional computer-generated representation of aspirin.
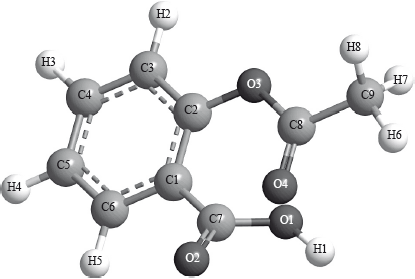
A third student measured selected bond lengths in aspirin, using this computer program and reported the following data.
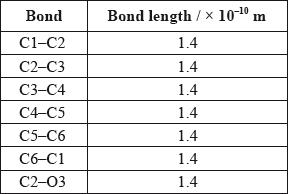
The following hypothesis was suggested by the student: “Since all the measured carbon-carbon bond lengths are equal, all the carbon-oxygen bond lengths must also be equal in aspirin. Therefore, the C8–O4 bond length must be 1.4 \( \times \) 10–10 m”. Comment on whether or not this is a valid hypothesis.
The other product of the reaction is ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\). Define an acid according to the Brønsted-Lowry theory and state the conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\).
Brønsted-Lowry definition of an acid:
Conjugate base of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\):
Markscheme
carboxylic acid / carboxyl;
ester;
Do not allow carbonyl / acid / ethanoate / formula(–COOH).
aryl group / benzene ring / phenyl;
\({M_{\text{r}}}{\text{(}}{{\text{C}}_7}{{\text{H}}_6}{{\text{O}}_3}{\text{)}} = {\text{138.13}}\);
\(n = \left( {\frac{{3.15}}{{138.13}} = } \right){\text{ }}2.28 \times {10^{ - 2}}{\text{ (mol)}}\);
Award [2] for the correct final answer.
\({M_{\text{r}}}{\text{(}}{{\text{C}}_9}{{\text{H}}_8}{{\text{O}}_4}{\text{)}} = 180.17\);
\(m = (180.17 \times 2.28 \times {10^{ - 2}} = ){\text{ }}4.11{\text{ (g)}}\);
Accept range 4.10–4.14
Award [2] for the correct final answer.
\({\text{(percentage yield}} = \frac{{2.50}}{{4.11}} \times 100 = ){\text{ }}60.8\% \);
Accept 60–61%.
3;
\({\text{(percentage uncertainty }} = \frac{{0.02}}{{2.50}} \times 100 = {\text{) }}0.80\% \);
Allow 0.8%
sample contaminated with ethanoic acid / aspirin not dry / impure sample;
Accept specific example of a systematic error.
Do not accept error in reading balance/weighing scale.
Do not accept yield greater than 100%.
hypothesis not valid/incorrect;
Accept any of the following for the second mark
C–O and C=O bond lengths will be different;
C2–O3 bond is longer than C8–O4 bond;
C8–O4 bond shorter than C2–O3 bond;
a CO single bond is longer than a CO double bond;
Accept C8–O4 is a double bond hence shorter.
Brønsted-Lowry definition of an acid
proton/H+/hydrogen ion donor;
Conjugate base of CH3COOH
\({\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{/C}}{{\text{H}}_3}{\text{CO}}_2^ - \);
Do not accept C2H3O2–/ethanoate.
Examiners report
In (a) Some candidates gave the correct three names of the functional groups; however some candidates gave answers such as alkene, ketone, aldehyde, ether, and carbonyl.
Candidates did not have problems determining the number of moles of salicylic acid used in (b) (i), although a few gave the answer with one significant digit only.
For (ii) the majority of candidates correctly used the value obtained in (i) to calculate the theoretical yield of aspirin.
In (iii) the percentage yield was calculated correctly in most cases.
The calculation of the percentage uncertainty (part (iv) proved to be a little more difficult, but many candidates gave the correct answer of 0.80%.
Part (v) was correctly answered by only a few candidates who stated that aspirin was contaminated or that the aspirin was not dry.
Nearly all the candidates correctly stated that the suggested hypothesis was not valid in (vi), giving the right reasons.
In (vii) most candidates gave the correct definition of an acid according to Brønsted-Lowry theory, although a few defined the acid according to Lewis theory. The conjugate base of the ethanoic acid was not always correct.
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:
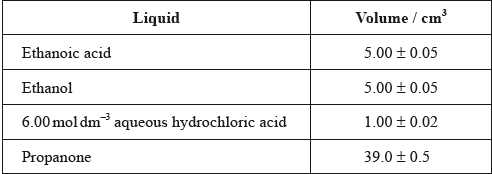
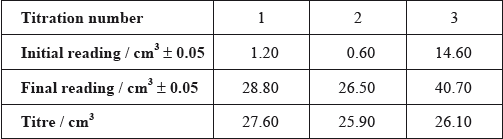
After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 2}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:
The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The hydrochloric acid does not appear in the balanced equation for the reaction. State its function.
Identify the liquid whose volume has the greatest percentage uncertainty.
(i) Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^{\text{3}}}\)).
(ii) Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
(iii) \({\text{23.00 c}}{{\text{m}}^{\text{3}}}\) of this \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the ethanoic acid in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the amount, in mol, of ethanoic acid present in the \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of final equilibrium mixture.
Referring back to your answer for part (a), calculate the percentage of ethanoic acid converted to ethyl ethanoate.
Deduce the equilibrium constant expression for the reaction.
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
Markscheme
\({\text{M(C}}{{\text{H}}_3}{\text{COOH)}}\left( { = (4 \times 1.01) + (2 \times 12.01) + (2 \times 16.00)} \right) = 60.06{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Accept 60 (g mol–1).
\({\text{mass (C}}{{\text{H}}_3}{\text{COOH)}}( = 5.00 \times 1.05) = 5.25{\text{ (g)}}\);
\(\frac{{5.25}}{{{\text{60.06}}}} = 0.0874{\text{ (mol)}}\);
Award [3] for correct final answer.
Accept 0.0875 (comes from using Mr = 60 g mol–1).
catalyst / OWTTE;
hydrochloric acid/HCl;
(i) \( \pm 0.1/0.10{\text{ (c}}{{\text{m}}^3}{\text{)}}\);
Do not accept without ±.
(ii) \({\text{26.00 (c}}{{\text{m}}^3}{\text{)}}\);
(iii) \(0.200 \times \frac{{23.00}}{{1000}} = 0.0046\);
\({\text{0.0046}} \times \frac{{{\text{50.0}}}}{{{\text{5.00}}}} = {\text{0.0460 (mol)}}\);
\(\frac{{0.0874 - 0.0460}}{{0.0874}} \times 100 = 47.4\% \);
\({\text{(}}{K_{\text{c}}} = {\text{)}}\frac{{{\text{[C}}{{\text{H}}_3}{\text{COO}}{{\text{C}}_2}{{\text{H}}_3}{\text{][}}{{\text{H}}_2}{\text{O]}}}}{{{\text{[}}{{\text{C}}_2}{{\text{H}}_5}{\text{OH][C}}{{\text{H}}_3}{\text{COOH]}}}}\);
Do not penalize minor errors in formulas.
Accept \({\text{(}}{K_{\text{c}}} = {\text{)}}\frac{{{\text{[}}ester{\text{][}}water{\text{]}}}}{{{\text{[}}ethanol / alcohol{\text{][(}}ethanoic{\text{)}} acid{\text{]}}}}\).
repeat the titration a day/week later (and result should be the same) / OWTTE;
Accept “concentrations/physical properties/macroscopic properties of the system do not change”.
enthalpy change/\(\Delta H\) for the reaction is (very) small / OWTTE;
decreases (the amount of ethanoic acid converted);
Accept “increases amount of ethanoic acid present at equilibrium” / OWTTE.
(adding product) shifts position of equilibrium towards reactants/LHS / increases the rate of the reverse reaction / OWTTE;
ethyl ethanoate/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\);
forms only weak hydrogen bonds (to water);
Allow “does not hydrogen bond to water” / “hydrocarbon sections too long” / OWTTE.
M2 can only be given only if M1 correct.
(large excess of) water will shift the position of equilibrium (far to the left) / OWTTE;
Accept any other chemically sound response, such as “dissociation of ethanoic acid would affect equilibrium”.
Examiners report
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Generally candidates found some elements of this question quite challenging but there were accessible marks of even the weakest candidates. The majority of students were able to determine the molar mass of ethanoic acid but some struggled to calculate the mass from the volume. Most candidates were able to identify the role of hydrochloric acid as a catalyst but some struggled to identify the liquid whose volume had the greatest uncertainty. Most candidates were able to calculate the absolute uncertainty of the titre but some lost a mark by omitting the \( + \)/\( - \) sign. Candidates did not identify the first titre as incongruent and simply averaged the three values which perhaps suggests limited experimental experience. Most students could determine an equilibrium constant expression, but many did not answer the question in (g) and did not suggest how the equilibrium could be established experimentally with many referring to the equal rate of the forward and backward reaction. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question asked and could not explain the small effect of temperature changes. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, many did not refer to its inability to form strong hydrogen bonds to water which was necessary for the mark. Quite a number of students came up with a valid reason why water would not be a suitable though some students appeared to have overlooked that the question asked for “one other reason” than that implied in (j).
Reaction kinetics can be investigated using the iodine clock reaction. The equations for two reactions that occur are given below.
Reaction A: \({{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
Reaction B: \({{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}\)
Reaction B is much faster than reaction A, so the iodine, \({{\text{I}}_{\text{2}}}\), formed in reaction A immediately reacts with thiosulfate ions, \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\), in reaction B, before it can react with starch to form the familiar blue-black, starch-iodine complex.
In one experiment the reaction mixture contained:
\(5.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of \({\text{2.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrogen peroxide \({\text{(}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{)}}\)
\(5.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of 1% aqueous starch
\(20.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid (\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\))
\(20.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.0100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium thiosulfate (\({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\))
\(50.0 \pm 0.1{\text{ c}}{{\text{m}}^{\text{3}}}\) of water with 0.0200 ± 0.0001 g of potassium iodide (KI) dissolved in it.
After 45 seconds this mixture suddenly changed from colourless to blue-black.
Calculate the amount, in mol, of KI in the reaction mixture.
Calculate the amount, in mol, of \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\) in the reaction mixture.
The concentration of iodide ions, \({{\text{I}}^ - }\), is assumed to be constant. Outline why this is a valid assumption.
For this mixture the concentration of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), can also be assumed to be constant. Explain why this is a valid assumption.
Explain why the solution suddenly changes colour.
Apart from the precision uncertainties given, state one source of error that could affect this investigation and identify whether this is a random error or a systematic error.
Calculate the total uncertainty, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of the volume of the reaction mixture.
The colour change occurs when \(1.00 \times {10^{ - 4}}{\text{ mol}}\) of iodine has been formed. Use the total volume of the solution and the time taken, to calculate the rate of the reaction, including appropriate units.
In a second experiment, the concentration of the hydrogen peroxide was decreased to \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) while all other concentrations and volumes remained unchanged. The colour change now occurred after 100 seconds. Explain why the reaction in this experiment is slower than in the original experiment.
In a third experiment, 0.100 g of a black powder was also added while all other concentrations and volumes remained unchanged. The time taken for the solution to change colour was now 20 seconds. Outline why you think the colour change occurred more rapidly and how you could confirm your hypothesis.
Explain why increasing the temperature also decreases the time required for the colour to change.
Markscheme
\(\left( {\frac{{0.0200}}{{166.00}} = } \right){\text{ }}0.000120/1.20 \times {10^{ - 4}}{\text{ (mol)}}\);
Accept 1.21 \( \times \) 10–4.
\((0.0050 \times 2.00 = ){\text{ }}0.010{\text{ (mol)}}/1.0 \times {10^{ - 2}}\);
KI/\({{\text{I}}^ - }\)/potassium iodide/iodide (ion) (rapidly) reformed (in second stage of reaction);
amount (in mol) of \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\)/hydrogen peroxide \( \gg \) amount (in mol) \({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{/}}{{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\)/sodium thiosulfate/ thiosulfate (ion);
Accept amount (in mol) of H2O2/hydrogen peroxide \( \gg \) amount (in mol) KI/I–/potassium iodide/iodide (ion).
Accept “[H2O2]/hydrogen peroxide is in (large) excess/high concentration”.
(at end of reaction) [\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\)] is only slightly decreased/virtually unchanged;
all \({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\)/sodium thiosulfate/\({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\)/thiosulfate consumed/used up;
Accept “iodine no longer converted to iodide”.
(free) iodine is formed / iodine reacts with starch / forms iodine-starch complex;
Random: synchronizing mixing and starting timing / (reaction) time / uncertainty of concentrations of solutions / temperature of solutions/room temperature;
OR
Systematic: liquid remaining in measuring cylinders / not all solid KI transferred / precision uncertainty of stopwatch / ability of human eye to detect colour change / parallax error;
Accept concentration of stock solution and human reaction time as systematic error.
Award M1 for correctly identifying a source of error and M2 for classifying it.
Accept other valid sources of error.
Do not accept “student making mistakes” / OWTTE.
\((5 \times 0.1) = ( \pm )0.5{\text{ }}({\text{c}}{{\text{m}}^3})\);
total volume \( = 0.100{\text{ }}({\text{d}}{{\text{m}}^3})/100{\text{ }}({\text{c}}{{\text{m}}^3})\);
\(\left( {{\text{change in concentration}} = \frac{{1.00 \times {{10}^{ - 4}}}}{{{\text{0.100}}}} = } \right){\text{ }}1.00 \times {10^{ - 3}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
\(\left( {{\text{rate}} = \frac{{1.00 \times {{10}^{ - 3}}}}{{45}} = } \right){\text{ }}2.2 \times {10^{ - 5}}\);
Award [3] for the correct final answer.
\({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}\);
fewer particles (per unit volume);
lower collision rate/collision frequency / less frequent collisions;
Do not accept “less collisions”.
acting as a catalyst / black powder reacts with thiosulfate ions / solid dissolves to give blue-black solution;
Accept any other valid suggestion which will make colour change more rapid.
For catalyst: amount/mass of black powder remains constant / no new/different products formed / activation energy decreased;
For other suggestions: any appropriate way to test the hypothesis;
Award [1] for valid hypothesis, [1] for appropriate method of testing the stated hypothesis.
particles have greater (average) kinetic energy;
Do not accept energy instead of kinetic energy.
more frequent collisions/collision frequency/number of collisions in a given time increases;
Do not accept “more collisions” unless “less collisions” penalized in (i).
greater proportion of particles have energy \( \ge\) activation energy;
Accept “particles have sufficient energy for collisions to be successful”.
Examiners report
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
This was a data based question based on quantitative chemistry and proved difficult for many candidates. Majority of candidates were able to gain almost full marks in parts (a) and (b). In part (c), many candidates failing to recognise that KI is rapidly reformed in the second stage of the reaction. In part (d), majority of candidates could not interpret the information correctly and hence lost two marks. Similarly, only 1 mark was obtained in part (e) where candidates recognized that iodine forms the starch-iodine complex. Many candidates managed the systematic and random errors in part (f). Calculation of uncertainty in part (g) was relatively well done by many candidates. In part (h), calculation of rate of reaction occasionally saw the erroneous use of volume in \({\text{c}}{{\text{m}}^{\text{3}}}\). In part (i), the candidates just repeated the stem of the question but obtained credit for the second mark for stating less frequent collisions. Part (j) was quite open ended and elicited a number of interesting responses (instead of acting as catalyst) whereas the suggested tests would not in fact confirm the hypothesis suggested. In part (k), the effect of increasing temperature on the rate of reaction proved easy for majority of candidates.
Consider the following reaction taking place at 375 °C in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} + {\text{S}}{{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\Theta } = - 84.5{\text{ kJ}}} \end{array}\]
A solution of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), is added to a solution of sodium iodide, NaI, acidified with hydrochloric acid, HCl. The yellow colour of the iodine, \({{\text{I}}_{\text{2}}}\), can be used to determine the rate of reaction.
\[{{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2NaI(aq)}} + {\text{2HCl(aq)}} \to {\text{2NaCl(aq)}} + {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\]
The experiment is repeated with some changes to the reaction conditions. For each of the changes that follow, predict, stating a reason, its effect on the rate of reaction.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\).
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
Graphing is an important method in the study of the rates of chemical reaction. Sketch a graph to show how the reactant concentration changes with time in a typical chemical reaction taking place in solution. Show how the rate of the reaction at a particular time can be determined.
The concentration of \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\) is increased at constant temperature.
The solution of NaI is prepared from a fine powder instead of large crystals.
Explain why the rate of a reaction increases when the temperature of the system increases.
Markscheme
\(({K_{\text{c}}}) = \frac{{{\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}}}{{{\text{[C}}{{\text{l}}_2}{\text{][S}}{{\text{O}}_2}{\text{]}}}}\);
Ignore state symbols.
Square brackets [ ] required for the equilibrium expression.
value of \({K_{\text{c}}}\) increases;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) increases;
decrease in temperature favours (forward) reaction which is exothermic;
Do not allow ECF.
no effect on the value of \({K_{\text{c}}}\) / depends only on temperature;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) decreases;
increase in volume favours the reverse reaction which has more gaseous moles;
Do not allow ECF.
no effect;
catalyst increases the rate of forward and reverse reactions (equally) / catalyst decreases activation energies (equally);
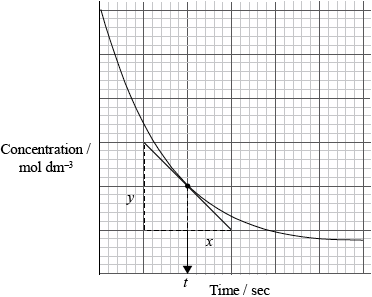
labelled axes (including appropriate units);
correctly drawn curve;
correctly drawn tangent;
rate equal to slope/gradient of tangent (at given time) / rate \( = \frac{y}{x}\) at time \(t\);
[3 max] for straight line graph or graph showing product formation.
increases rate of reaction;
molecules (of \({{\text{H}}_2}{{\text{O}}_2}\)) collide more frequently / more collisions per unit time;
No ECF here.
no effect / (solution) remains unchanged;
solid NaI is not reacting / aqueous solution of NaI is reacting / surface area of NaI is not relevant in preparing the solution / OWTTE;
kinetic energy/speed of reacting molecules increases;
frequency of collisions increases per unit time;
greater proportion of molecules have energy greater than activation energy/\({E_{\text{a}}}\);
Accept more energetic collisions.
Examiners report
This was the most popular question in Section B and there was a generally pleasing level of performance. In (a)(i) most candidates were able to correctly deduce the equilibrium constant.
In (ii) most candidates realized the exothermic reaction would be favoured, and gained full marks for their explanation. However, some candidates seemed not to appreciate that the specified temperature of 300 °C was lower than the original, and so based their answers on a temperature increase.
In (iii) most forgot to mention the word gaseous when talking about the particles and many forgot that \({K_{\text{c}}}\) is only affected by temperature.
In (iv) candidates correctly stated that concentration would not change and stated that reaction rates of both forward and reverse reactions would be affected equally. However, some answered ‘the addition of a catalyst does not affect \({K_{\text{c}}}\) or the position of equilibrium’ which did not answer the question and scored no marks as they had not commented on the concentration of \({\text{SOC}}{{\text{l}}_{\text{2}}}\).
For (b), although most students were able to correctly sketch the reactant concentration / time graph by labeling the axes and drawing an appropriate curve, some candidates incorrectly read the question and sketched product / concentration time curve. Drawing a tangent to determine the rate was not well known and only some were able to describe how the rate at a particular instant could be determined from the tangent to the curve.
In (c), most scored the marks in (i) and were able to correctly describe the effect of concentration on rate in terms of collision theory, although some forgot to mention the frequency of the collisions just stating there would be more.
In part (ii), most candidates assumed that the rate would increase with surface area of the solute, and few realized that once the sodium iodide was in solution then the particle size of the solid used to make it was not relevant as it is the solution which reacts.
Part (d) was well answered but some candidates lost marks due to imprecise responses. For example it is the kinetic energy that increases with temperature, not energy. Also there were some errors such as the omission of the idea of frequency when referring to collisions and the belief that an increase in temperature caused a decrease in activation energy.
A student used a pH meter to measure the pH of different samples of water at 298 K.
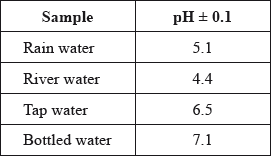
Use the data in the table to identify the most acidic water sample.
Calculate the percentage uncertainty in the measured pH of the rain water sample.
Determine the ratio of \({\text{[}}{{\text{H}}^ + }{\text{]}}\) in bottled water to that in rain water.
\[\frac{{[{H^ + }]{\text{ }}in{\text{ }}bottled{\text{ }}water}}{{[{H^ + }]{\text{ }}in{\text{ }}rain{\text{ }}water}}\]
The acidity of non-polluted rain water is caused by dissolved carbon dioxide. State an equation for the reaction of carbon dioxide with water.
Markscheme
river (water);
\(\left( {\frac{{0.1}}{{5.1}} \times 100 = } \right){\text{ }}2\% \);
recognition that values differ by 2 Ph units / calculation of both \({\text{[}}{{\text{H}}^ + }{\text{]}}\) values;
\({\text{(ratio)}} = 1:100/{10^{ - 2}}/0.01/\frac{1}{{100}}\);
Award [2] for correct final answer.
Award [1 max] for 100:1/100/102.
\({\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}^ + }/{\text{C}}{{\text{O}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}} \rightleftharpoons {\text{HCO}}_3^ - + {{\text{H}}_3}{{\text{O}}^ + }/{\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}} \rightleftharpoons {{\text{H}}_2}{\text{C}}{{\text{O}}_3}\);
Do not penalize missing reversible arrow.
Do not accept equations with the carbonate ion as a product.
Examiners report
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Parts (a) and (b) were correctly answered by the majority of candidates, the most common mistake being to assume that (b) referred to the sample identified in (a). Part (c) was rather more challenging and students frequently used the ratio of the pH rather than the ratio of the \({\text{[}}{{\text{H}}^ + }{\text{]}}\). Part (d) should have been very straightforward, but was often poorly answered with some innovative products. The absence of an equilibrium arrow was not penalised, but if it had been many students would have lost a mark.
Ethanedioic acid is a diprotic acid. A student determined the value of x in the formula of hydrated ethanedioic acid, \({\text{HOOC}}\)–\({\text{COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\), by titrating a known mass of the acid with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of \({\text{NaOH(aq)}}\).
0.795 g of ethanedioic acid was dissolved in distilled water and made up to a total volume of \({\text{250 c}}{{\text{m}}^{\text{3}}}\) in a volumetric flask.
\({\text{25 c}}{{\text{m}}^{\text{3}}}\) of this ethanedioic acid solution was pipetted into a flask and titrated against aqueous sodium hydroxide using phenolphthalein as an indicator.
The titration was then repeated twice to obtain the results below.
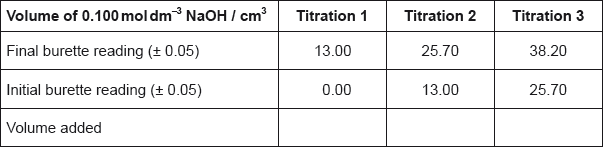
State the uncertainty of the volume of NaOH added in \({\text{c}}{{\text{m}}^{\text{3}}}\).
Calculate the average volume of NaOH added, in \({\text{c}}{{\text{m}}^{\text{3}}}\), in titrations 2 and 3, and then calculate the amount, in mol, of NaOH added.
(i) The equation for the reaction taking place in the titration is:
\({\text{HOOC}}\)−\({\text{COOH(aq)}} + {\text{2NaOH(aq)}} \to {\text{NaOOC}}\)−\({\text{COONa(aq)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\)
Determine the amount, in mol, of ethanedioic acid that reacts with the average volume of \({\text{NaOH(aq)}}\).
(ii) Determine the amount, in mol, of ethanedioic acid present in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the original solution.
(ii) Determine the molar mass of hydrated ethanedioic acid.
(iv) Determine the value of x in the formula \({\text{HOOC}}\)−\({\text{COOH}} \bullet {\text{x}}{{\text{H}}_{\text{2}}}{\text{O}}\).
Identify the strongest intermolecular force in solid ethanedioic acid.
Deduce the Lewis (electron dot) structure of ethanedioic acid, HOOC−COOH.
Markscheme
\(( \pm )0.10{\text{ (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Accept ±0.1 (cm3).
Accept (±)0.09 (cm3) (based on more accurate method of calculating propagation of uncertainties).
\(\left( {\frac{{12.70 + 12.50}}{2} = } \right)12.60{\text{ (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\((0.01260 \times 0.100 = )1.26 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [2] for correct final answer.
(i) \(\left( {\frac{{1.26 \times {{10}^{ - 3}}}}{2} = } \right)6.30 \times {10^{ - 4}}{\text{ (mol)}}\);
(ii) \((6.30 \times {10^{ - 4}} \times 10 = )6.30 \times {10^{ - 3}}{\text{ (mol)}}\);
(iii) \(\left( {\frac{{0.795}}{{6.30 \times {{10}^{ - 3}}}} = } \right)126{\text{ (gmo}}{{\text{l}}^{ - 1}})\);
(iv) \({M_{\text{r}}}{\text{(}}{{\text{C}}_2}{{\text{H}}_2}{{\text{O}}_4}{\text{)}} = 90.04\) and \({M_{\text{r}}}{\text{(}}{{\text{H}}_2}{\text{O)}} = 18.02\);
\({\text{x}} = 2\);
Accept integer values for Mr’s of 90 and 18 and any reasonable calculation.
Award [1 max] if no working shown.
hydrogen bonding;
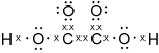 ;
;
Mark cannot be scored if lone pairs are missing on oxygens.
Accept any combination of lines, dots or crosses to represent electron pairs.
Examiners report
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there were some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
This beginning of this question to state the uncertainty and to calculate the average volume added were well done and most students could also calculate the number of moles added. However, many candidates began to lose marks from this point onwards. Some could identify the ratio and correctly state the moles of ethanedioic acid, but fewer realized they needed to multiply 10 to get back to the original solution. The next step to calculate the \({M_{\text{r}}}\) was only correctly completed by a handful of students. Those that were correct with the molar mass always could calculate the moles of water, many students just guessed an answer though.
The intermolecular force was correctly described as hydrogen bonding, however there was some instances when it seemed unclear whether students realized this was between molecules and instead they seemed to suggest it was a bond between hydrogen and oxygen in the molecule. Some candidates could correctly draw the Lewis structure but a number of those lost marks for omitting the lone pairs on oxygen.
The graph below shows pressure and volume data collected for a sample of carbon dioxide gas at 330 K.
Draw a best-fit curve for the data on the graph.
Deduce the relationship between the pressure and volume of the sample of carbon dioxide gas.
Use the data point labelled X to determine the amount, in mol, of carbon dioxide gas in the sample.
Markscheme
smooth curve through the data;
Do not accept a curve that passes through all of the points or an answer that joins the points using lines.
inversely proportional / \({\text{V}}\alpha \frac{{\text{1}}}{{\text{p}}}\) / \({\text{P}}\alpha \frac{{\text{1}}}{{\text{V}}}\) ;
Accept inverse/negative correlation/relationship.
Do not accept \({\text{V}} = \frac{{\text{1}}}{{\text{p}}}\) / \({\text{P}} = \frac{{\text{1}}}{{\text{V}}}\) or descriptions like “one goes up as other goes down” / OWTTE.
\(p = 21 \times {10^5}/2.1 \times {10^6}{\text{ (Pa)}}/2.1 \times {10^3}{\text{ (kPa)}}\) and
\(V = 50 \times {10^{ - 6}}/5.0 \times {10^{ - 5}}{\text{ }}({{\text{m}}^3})/5.0 \times {10^{ - 2}}{\text{ }}({\text{d}}{{\text{m}}^3})\);
\(\left( {n = \frac{{pV}}{{RT}} = } \right){\text{ }}\frac{{2.1 \times {{10}^6} \times 5.0 \times {{10}^{ - 5}}}}{{8.31 \times 330}}\);
\(n = 0.038{\text{ (mol)}}\);
Award [3] for correct final answer.
For M3 apply ECF for correct computation of the equation the student has written, unless more than one mistake is made prior this point.
Examiners report
Almost all candidates gained the mark for drawing a best-fit curve through the data points on the graph, though some insisted in trying to put a straight line through obviously non-linear data. Many students identified the inverse proportionality of pressure and volume in Part (b), though the terminology often lacked precision. Most students could identify the correct equation to use in Part (c) in order to calculate the amount of gas from the specified data point, though quite often they had problems with units, either as a result of incorrectly reading the axis on the graph or as a result of conversion.
Almost all candidates gained the mark for drawing a best-fit curve through the data points on the graph, though some insisted in trying to put a straight line through obviously non-linear data. Many students identified the inverse proportionality of pressure and volume in Part (b), though the terminology often lacked precision. Most students could identify the correct equation to use in Part (c) in order to calculate the amount of gas from the specified data point, though quite often they had problems with units, either as a result of incorrectly reading the axis on the graph or as a result of conversion.
Almost all candidates gained the mark for drawing a best-fit curve through the data points on the graph, though some insisted in trying to put a straight line through obviously non-linear data. Many students identified the inverse proportionality of pressure and volume in Part (b), though the terminology often lacked precision. Most students could identify the correct equation to use in Part (c) in order to calculate the amount of gas from the specified data point, though quite often they had problems with units, either as a result of incorrectly reading the axis on the graph or as a result of conversion.
A student carried out an experiment to determine the concentration of a hydrochloric acid solution and the enthalpy change of the reaction between aqueous sodium hydroxide and this acid by thermometric titration.
She added \({\text{5.0 c}}{{\text{m}}^{\text{3}}}\) portions of hydrochloric acid to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution in a glass beaker until the total volume of acid added was \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\), measuring the temperature of the mixture each time. Her results are plotted in the graph below.

The initial temperature of both solutions was the same.
By drawing appropriate lines, determine the volume of hydrochloric acid required to completely neutralize the \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of sodium hydroxide solution.
Determine the concentration of the hydrochloric acid, including units.
Determine the change in temperature, \(\Delta T\).
Calculate the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of hydrochloric acid and sodium hydroxide solution.
The accepted theoretical value from the literature of this enthalpy change is \( - 58{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Calculate the percentage error correct to two significant figures.
Suggest the major source of error in the experimental procedure and an improvement that could be made to reduce it.
Markscheme
drawing best-fit straight lines to show volume;
There should be approximately the same number of points above and below for both lines.
\({\text{27.0 (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Accept any value in the range 26.0 to 28.0 (cm3) if consistent with student’s annotation on the graph.
Accept ECF for volumes in the range 27.0–30.0 cm3 if it corresponds to maximum temperature of line drawn.
Volumes should be given to one decimal place.
\({\text{[HCl]}} = \frac{{1.00 \times 0.0250}}{{0.0270}}\);
\( = 0.926{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
Volume of 26.0 gives [HCl] = 0.962 mol dm–3. Volume of 28.0 gives [HCl] = 0.893 mol dm–3
Award [2] for correct final answer with units.
Award [1 max] for correct concentration without units.
Accept M, mol L–1, mol/dm3 as units.
\((30.2 - 25.0 = )( + )5.2{\rm{ (^\circ C/K)}}\);
Any accepted value must be consistent with student’s annotation on the graph but do not accept \(\Delta T < 5.1\).
Accept \(( + )5.6{\rm{ (^\circ C/K) }}\) (ie, taking into account heat loss and using T when volume = 0.0 cm3).
\({\text{Q}} = (m \times c \times \Delta T = (25.0 + 27.0) \times 4.18 \times 5.2 = 1130.272{\text{ J}} = )1.13{\text{ (kJ)}}\);
\(n = (1.00 \times 0.0250 = )0.0250{\text{ (mol)}}\);
\(\Delta H = \left( { - \frac{Q}{n} = - 45210.88{\text{ J}}\,{\text{mo}}{{\text{l}}^{ - 1}} = } \right) - 45{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for +45 (kJ mol –1).
Apply ECF for M3 even if both m and \(\Delta T\) are incorrect in M1.
Accept use of c = 4.2 Jg–1K–1.
\(\left( {\left| {\frac{{ - 45 - ( - 58)}}{{( - 58)}}} \right| \times 100 = } \right)22(\% )\);
Answer must be given to two significant figures.
Ignore sign.
heat losses;
better (thermal) insulation / using a polystyrene cup / putting a lid on the beaker;
Accept other suitable methods for better thermal insulation, but do not accept just "use a calorimeter" without reference to insulation.
Examiners report
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
Some teachers commented that thermometric titrations are not listed in the syllabus nor are they included as prescribed experiments for the new guide. A similar question was asked in a past examination and thermometric titrations are covered in Topic 5. The intention is that any data based questions should be accessible to all students, who have the appropriate practical experience. It is not intended that such questions will be constrained to experiments on this list. Most candidates were not able to access the first mark with by construction of lines of best fit. Some drew a 'dot to dot' curve, but with most just providing a construction line dropping down from the maximum point on the graph, which did allow them to access the second mark. There was some transferred error for 1aii), but many were not able to carry out the calculation. Scoring for the temperature difference was dependent upon on the candidate's annotations, with a few extending the line of best fit back to the y axis. In the calculation of enthalpy change, the total mass of the solutions was often incorrect, but some salvaged the subsequent marks. The calculation of percentage error was generally done well, but a good third of the candidates failed to read the question stem and did not give the answer to two significant figures. The concept of heat loss in the experiment was well understood, but the solution was very often too vague.
The standard enthalpy change of three combustion reactions are given below.
\[\begin{array}{*{20}{l}} {{{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {{\text{H}}_2}{\text{O(l)}}}&{\Delta H = - 286{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}}&{\Delta H = - 2219{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \\ {{\text{C(s)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}}}&{\Delta H = - 394{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Determine the change in enthalpy, \(\Delta H\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the formation of propane in the following reaction.
\({\text{3C(s)}} + {\text{4}}{{\text{H}}_2}{\text{(g)}} \to {{\text{C}}_3}{{\text{H}}_8}{\text{(g)}}\)
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\). Define the term activation energy, \({E_{\text{a}}}\).
Sketch two Maxwell–Boltzmann energy distribution curves for a fixed amount of gas at two different temperatures, \({T_{\text{1}}}\) and \({T_2}{\text{ }}({T_2} > {T_1})\) and label both axes.

Markscheme
\({\text{4}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {\text{3C}}{{\text{O}}_2}{\text{(g)}} \to {{\text{C}}_3}{{\text{H}}_8}{\text{(g)}} + {\text{5}}{{\text{O}}_2}{\text{(g)}}\) \(\Delta H = + {\text{2219 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\({\text{4}}{{\text{H}}_2}{\text{(g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{4}}{{\text{H}}_2}{\text{O(l)}}\): \(\Delta H = \left( {{\text{(}} - {\text{286)(4)}} = } \right){\text{ }} - {\text{1144 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\({\text{3C(s)}} + {\text{3}}{{\text{O}}_2}{\text{(g)}} \to {\text{3C}}{{\text{O}}_2}{\text{(g)}}\): \(\Delta H = \left( {{\text{(}} - {\text{394)(3)}} = } \right){\text{ }} - {\text{1182 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\(\Delta H = \left( {{\text{(}} - {\text{286)(4)}} + {\text{(}} - {\text{394)(3)}} + {\text{(}} + {\text{2219)}} = } \right){\text{ }} - {\text{107 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [4] for correct final answer.
minimum energy needed (by reactants/colliding particles) to react/start/initiate a reaction / for a successful collision;
Allow energy difference between reactants and transition state.
x-axis label: (kinetic) energy/(K)E and y-axis label: fraction of molecules/particles / probability density;
Allow velocity/speed for x-axis.
Allow frequency / number of molecules/particles or (kinetic) energy distribution for y-axis.
correct shape of a Maxwell–Boltzmann energy distribution curve;
Do not award mark if curve is symmetric, does not start at zero or if it crosses x-axis.
two curves represented with second curve for \({T_2} > {T_1}\) to right of first curve, lower
peak than first curve and after the curves cross \({T_2}\) curve needs to be above \({T_1}\) curve;
Examiners report
In contrast, question 2 a) which involved Hess’s Law calculation, was answered correctly by candidates of all capabilities.
The definition of activation energy in part b) was reasonably well answered, with some candidates losing marks for omitting the word minimum from their response. However, it is disappointing that even very good candidates sometimes fail to score marks for definitions.
Several candidates sketched very clear, correct Maxwell-Boltzmann curves in part c). Most scored at least 1 mark for this question. Some did not know what labels to put on the axes. Some did not realise that the area under the curves represents the total number of particles so as temperature increases the peak of the curve shifts to the right and is lower than the peak at the lower temperature.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
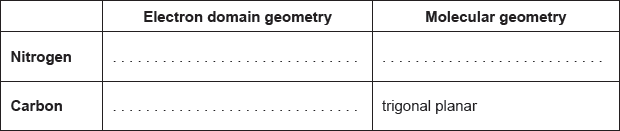
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) \( \rightleftharpoons \) (H2N)2CO(g) + H2O(g) ΔH < 0
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
The mass spectrum of urea is shown below.
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Markscheme
molar mass of urea «= 4 × 1.01 + 2 × 14.01 + 12.01 + 16.00» = 60.07 «g mol–1»
«% nitrogen = \(\frac{{2 \times 14.01}}{{60.07}}\) × 100 =» 46.65 «%»
Award [2] for correct final answer.
Award [1 max] for final answer not to two decimal places.
[2 marks]
«cost» increases AND lower N% «means higher cost of transportation per unit of nitrogen»
OR
«cost» increases AND inefficient/too much/about half mass not nitrogen
Accept other reasonable explanations.
Do not accept answers referring to safety/explosions.
[1 mark]
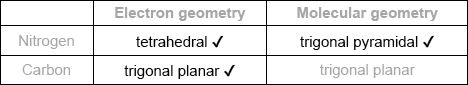
Note: Urea’s structure is more complex than that predicted from VSEPR theory.
[3 marks]
n(KNCO) «= 0.0500 dm3 × 0.100 mol dm–3» = 5.00 × 10–3 «mol»
«mass of urea = 5.00 × 10–3 mol × 60.07 g mol–1» = 0.300 «g»
Award [2] for correct final answer.
[2 marks]
«Kc» decreases AND reaction is exothermic
OR
«Kc» decreases AND ΔH is negative
OR
«Kc» decreases AND reverse/endothermic reaction is favoured
[1 mark]
Any one of:
urea has greater molar mass
urea has greater electron density/greater London/dispersion
urea has more hydrogen bonding
urea is more polar/has greater dipole moment
Accept “urea has larger size/greater van der Waals forces”.
Do not accept “urea has greater intermolecular forces/IMF”.
[1 mark]
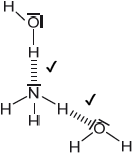
Award [1] for each correct interaction.
If lone pairs are shown on N or O, then the lone pair on N or one of the lone pairs on O MUST be involved in the H-bond.
Penalize solid line to represent H-bonding only once.
[2 marks]
2(H2N)2CO(s) + 3O2(g) → 4H2O(l) + 2CO2(g) + 2N2(g)
correct coefficients on LHS
correct coefficients on RHS
Accept (H2N)2CO(s) + \(\frac{3}{2}\)O2(g) → 2H2O(l) + CO2(g) + N2(g).
Accept any correct ratio.
[2 marks]
60: CON2H4+
44: CONH2+
Accept “molecular ion”.
[2 marks]
3450 cm–1: N–H
1700 cm–1: C=O
Do not accept “O–H” for 3450 cm–1.
[2 marks]
1
[1 mark]
Examiners report
A student decided to determine the molecular mass of a solid monoprotic acid, HA, by titrating a solution of a known mass of the acid.
The following recordings were made.
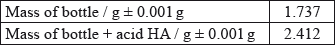
Calculate the mass of the acid and determine its absolute and percentage uncertainty.
This known mass of acid, HA, was then dissolved in distilled water to form a \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) solution in a volumetric flask. A \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) sample of this solution reacted with \({\text{12.1 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaOH solution. Calculate the molar mass of the acid.
The percentage composition of HA is 70.56% carbon, 23.50% oxygen and 5.94% hydrogen. Determine its empirical formula.
A solution of HA is a weak acid. Distinguish between a weak acid and a strong acid.
Describe an experiment, other than measuring the pH, to distinguish HA from a strong acid of the same concentration and describe what would be observed.
Markscheme
0.675 (g) ± 0.002 (g);
Percentage uncertainty: 0.3%;
Accept answers correct to one, two or three significant figures for percentage uncertainty.
In 25.0 cm3: \({n_{{\text{HA}}}} = 1.21 \times {10^{ - 3}}{\text{ (mol)}}\);
In 100 cm3: \({n_{{\text{HA}}}} = 4.84 \times {10^{ - 3}}{\text{ (mol)}}\);
\({\text{M }}\left( { = \frac{{0.675}}{{4.84 \times {{10}^{ - 3}}}}} \right) = 139{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Accept suitable alternative methods.
\({n_{\text{C}}}:{\text{ }}\left( {\frac{{70.56}}{{12.01}} = } \right){\text{ }}5.88\) and \({n_{\text{O}}}:{\text{ }}\left( {\frac{{23.50}}{{16}} = } \right){\text{ }}1.47\) and \({n_{\text{H}}}:{\text{ }}\left( {\frac{{5.94}}{{1.01}} = } \right){\text{ }}5.88\)
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{4}}}{\text{O}}\);
Award [2] for correct final answer.
Accept answers using integer values of molar mass.
weak acids partially dissociated/ionized and strong acids completely dissociated/ionized (in solution/water) / OWTTE;
strong acids have greater electrical conductivity / weak acids have lower electrical conductivity;
OR
adding a reactive metal / carbonate / hydrogen carbonate;
Accept correct example.
stronger effervescence with strong acids / weaker with weak acids / OWTTE;
OR
adding a strong base;
Accept correct example.
strong acid would increase more in temperature / weak acids increase less in temperature;
Examiners report
Many students lost easy marks as they forgot to propagate uncertainties.
Many candidates struggled with the concept of mole and the dilution factor added to the difficulty.
Most students determined the empirical formula correctly.
Weak and strong acids were generally correctly defined, though sometimes they were defined in terms of pH.
The conductivity test appeared frequently and was well described. Many candidates used a strong based, but then went on to describe a titration method.
Airbags are an important safety feature in vehicles. Sodium azide, potassium nitrate and silicon dioxide have been used in one design of airbag.

Sodium azide, a toxic compound, undergoes the following decomposition reaction under certain conditions.
\[{\text{2Na}}{{\text{N}}_{\text{3}}}{\text{(s)}} \to {\text{2Na(s)}} + {\text{3}}{{\text{N}}_{\text{2}}}{\text{(g)}}\]
Two students looked at data in a simulated computer-based experiment to determine the volume of nitrogen generated in an airbag.
Using the simulation programme, the students entered the following data into the computer.
The chemistry of the airbag was found to involve three reactions. The first reaction involves the decomposition of sodium azide to form sodium and nitrogen. In the second reaction, potassium nitrate reacts with sodium.
\[{\text{2KN}}{{\text{O}}_3}{\text{(s)}} + {\text{10Na(s)}} \to {{\text{K}}_2}{\text{O(s)}} + {\text{5N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{N}}_2}{\text{(g)}}\]
An airbag inflates very quickly.
Sodium azide involves ionic bonding, and metallic bonding is present in sodium. Describe ionic and metallic bonding.
State the number of significant figures for the temperature, mass and pressure data.
T:
m:
p:
Calculate the amount, in mol, of sodium azide present.
Determine the volume of nitrogen gas, in \({\text{d}}{{\text{m}}^{\text{3}}}\), produced under these conditions based on this reaction.
Suggest why it is necessary for sodium to be removed by this reaction.
The metal oxides from the second reaction then react with silicon dioxide to form a silicate in the third reaction.
\[{{\text{K}}_2}{\text{O(s)}} + {\text{N}}{{\text{a}}_2}{\text{O(s)}} + {\text{Si}}{{\text{O}}_2}{\text{(s)}} \to {\text{N}}{{\text{a}}_2}{{\text{K}}_2}{\text{Si}}{{\text{O}}_4}{\text{(s)}}\]
Draw the structure of silicon dioxide and state the type of bonding present.
Structure:
Bonding:
It takes just 0.0400 seconds to produce nitrogen gas in the simulation. Calculate the average rate of formation of nitrogen in (b) (iii) and state its units.
The students also discovered that a small increase in temperature (e.g. 10 °C) causes a large increase (e.g. doubling) in the rate of this reaction. State one reason for this.
Markscheme
Ionic:
(electrostatic) attraction between oppositely charged ions/cations and anions/positive and negative ions;
Do not accept answers such as compounds containing metal and non-metal are ionic.
Metallic:
(electrostatic attraction between lattice of) positive ions/cations/nuclei and delocalized electrons / (bed of) positive ions/cations/nuclei in sea of electrons / OWTTE;
T: 4 and m: 3 and p: 3;
\(n = (65.0/65.02) = 1.00{\text{ (mol)}}\);
No penalty for using whole number atomic masses.
\(n{\text{(}}{{\text{N}}_{\text{2}}}{\text{)}} = \left( {\frac{3}{2} \times 1.00 = } \right){\text{ }}1.50{\text{ (mol)}}\);
\(T = \left( {(25.00 + 273.15) = } \right){\text{ }}298.15{\text{ K}}/(25.00 + 273) = 298{\text{ K}}\);
\(p = 1.08 \times 1.01 \times {10^5}{\text{ Pa}}/1.08 \times 1.01 \times {10^2}{\text{ kPa}}/1.09 \times {10^5}{\text{ Pa}}/1.09 \times {10^2}{\text{ kPa}}\);
\(V = \frac{{nRT}}{p} = \frac{{({{10}^3})(1.50)(8.31)(298.15/298)}}{{(1.08 \times 1.01 \times {{10}^5})}} = 34.1{\text{ (d}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Award [4] for correct final answer.
Award [3 max] for 0.0341 (dm3) or 22.7 (dm3).
Award [3 max] for 34.4 (dm3).
Award [2 max] for 22.9 (dm3).
Award [2 max] for 0.0227 (dm3).
Award [2 max] for 0.034 (dm3).
sodium could react violently with any moisture present / sodium is (potentially) explosive / sodium (is dangerous since it is flammable when it) forms hydrogen on contact with water / OWTTE;
Do not accept answers such as sodium is dangerous or sodium is too reactive.
Structure:
drawing of giant structure showing tetrahedrally arranged silicon;
Minimum information required for mark is Si and 4 O atoms, in a tetrahedral arrangement (not 90° bond angles) but with each of the 4 O atoms showing an extension bond.
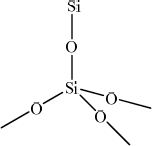
Bonding:
(giant/network/3D) covalent;
\(\left( {\frac{{34.1}}{{0.0400}}} \right) = 853{\text{ d}}{{\text{m}}^{\text{3}}}{{\text{s}}^{ - 1}}/\left( {\frac{{1.50}}{{0.0400}}} \right) = 37.5{\text{ mol}}\,{{\text{s}}^{ - 1}}\);
Accept 851 dm3s–1.
Units required for mark.
more energetic collisions / more species have energy \( \geqslant {E_{\text{a}}}\);
Allow more frequent collisions / species collide more often.
Examiners report
Question 1 tested a number of concepts and very few students were able to gain all the marks available. Part (a) was fairly well done and students could explain ionic and metallic bonding although weak students did not explain the bonding but simply stated that ionic was between metal and non metal etc.
Surprisingly in part (b) (i) a number of students could not state the number of significant figures and many stated that 25.00 was 2 SF instead of 4.
Part (b) (ii) required the calculation of the amount of substance in moles, and was generally well done although some did not realise the value was in kg and so had a value 1000 times too small.
In part (b) (iii) a number of students lost marks for forgetting to convert temperature or pressure and also to multiply the amount by 1.5. Also many forgot to convert the pressure into kPa if they wanted their answer in \({\text{d}}{{\text{m}}^{\text{3}}}\). However, most students could obtain at least one of the marks available.
In part (c) (i) many did not relate the removal of sodium to the potential for it to react with water and instead gave a far too vague of answer that it was reactive. However, the very best students were able to answer this hypothesis type question and stated that sodium reacts with water. This proved a good discriminator at the top end of the candidature.
Part (c)(ii) was very poorly answered and the majority of students believed that \({\text{Si}}{{\text{O}}_{\text{2}}}\) had a similar structure to \({\text{C}}{{\text{O}}_{\text{2}}}\). The very few students that drew a giant structure often did not then show a tetrahedral arrangement of the atoms, however most did realise that the bonding was covalent.
Part (d) was generally well answered and most students calculated a rate from their results although some lost the mark for incorrect or absent units.
Most students could then successfully explain why the rate increased with temperature. However a minority forgot to refer to time (i.e. more frequent) in relation to collisions.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
Suggest how the change of iodine concentration could be followed.
A student produced these results with [H+] = 0.15 mol\(\,\)dm−3. Propanone and acid were in excess and iodine was the limiting reagent.
Determine the relative rate of reaction when [H+] = 0.15 mol\(\,\)dm−3.
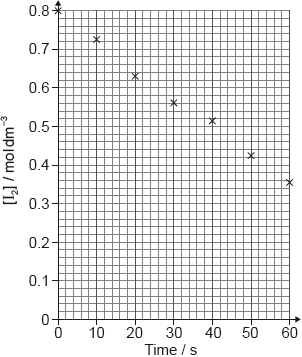
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
State and explain the relationship between the rate of reaction and the concentration of acid.
Markscheme
use a colorimeter/monitor the change in colour
OR
take samples AND quench AND titrate «with thiosulfate»
Accept change in pH.
Accept change in conductivity.
Accept other suitable methods.
Method must imply “change”.
[1 mark]
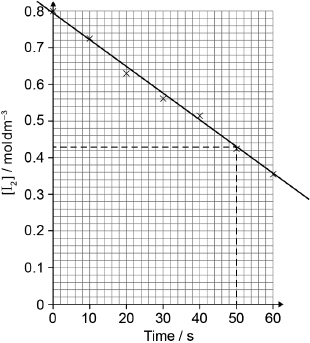
best fit line
relative rate of reaction = «\(\frac{{ - \Delta y}}{{\Delta x}} = \frac{{ - \left( {0.43 - 0.80} \right)}}{{50}}\) =» 0.0074/7.4 x 10−3
Best fit line required for M1.
M2 is independent of M1.
Accept range from 0.0070 to 0.0080.
[2 marks]
Relationship:
rate of reaction is «directly» proportional to [H+]
OR
rate of reaction \(\alpha \) [H+]
Explanation:
more frequent collisions/more collisions per unit of time «at greater concentration»
Accept "doubling the concentration doubles the rate".
Do not accept “rate increases as concentration increases”.
Do not accept collisions more likely.
[2 marks]
Examiners report
Two groups of students (Group A and Group B) carried out a project* on the chemistry of some group 7 elements (the halogens) and their compounds.
* Adapted from J Derek Woollins, (2009), Inorganic Experiments and Open University, (2008), Exploring the Molecular World.
In the first part of the project, the two groups had a sample of iodine monochloride (a corrosive brown liquid) prepared for them by their teacher using the following reaction.
\[{{\text{I}}_{\text{2}}}{\text{(s)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \to {\text{2ICl(l)}}\]
The following data were recorded.
The students reacted ICl(l) with CsBr(s) to form a yellow solid, \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), as one of the products. \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\) has been found to produce very pure CsCl(s) which is used in cancer treatment.
To confirm the composition of the yellow solid, Group A determined the amount of iodine in 0.2015 g of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\) by titrating it with \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\). The following data were recorded for the titration.
(i) State the number of significant figures for the masses of \({{\text{I}}_{\text{2}}}{\text{(s)}}\) and ICl(l).
\({{\text{I}}_{\text{2}}}{\text{(s)}}\):
ICl (l):
(ii) The iodine used in the reaction was in excess. Determine the theoretical yield, in g, of ICl(l).
(iii) Calculate the percentage yield of ICl(l).
(iv) Using a digital thermometer, the students discovered that the reaction was exothermic. State the sign of the enthalpy change of the reaction, \(\Delta H\).
Although the molar masses of ICl and \({\rm{B}}{{\rm{r}}_2}\) are very similar, the boiling point of ICl is 97.4 °C and that of \({\rm{B}}{{\rm{r}}_2}\) is 58.8 °C. Explain the difference in these boiling points in terms of the intermolecular forces present in each liquid.
(i) Calculate the percentage of iodine by mass in \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), correct to three significant figures.
(ii) State the volume, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\) used in the titration.
(iii) Determine the amount, in mol, of \({\text{0.0500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\) added in the titration.
(iv) The overall reaction taking place during the titration is:
\[{\text{CsICl(s)}} + {\text{2N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3}{\text{(aq)}} \to {\text{NaCl(aq)}} + {\text{N}}{{\text{a}}_2}{{\text{S}}_4}{{\text{O}}_6}{\text{(aq)}} + {\text{CsCl(aq)}} + {\text{NaI(aq)}}\]
Calculate the amount, in mol, of iodine atoms, I, present in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\).
(v) Calculate the mass of iodine, in g, present in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}\)
(vi) Determine the percentage by mass of iodine in the sample of \({\text{CsIC}}{{\text{l}}_{\text{2}}}{\text{(s)}}\), correct to three significant figures, using your answer from (v).
Markscheme
(i) I2(s): four/4 and ICl(l): three/3;
(ii) \(n{\text{(C}}{{\text{l}}_2}{\text{)}} = {\text{ }}\left( {\frac{{2.24}}{{2 \times 35.45}} = } \right){\text{ }}0.0316/3.16 \times {10^{ - 2}}{\text{ (mol)}}\);
Allow answers such as 3.2 \( \times \) 10–2/0.032/3.15 \( \times \) 10–2/0.0315 (mol).
\(n{\text{(ICl)}} = 2 \times 0.0316/0.0632/6.32 \times {10^{ - 2}}{\text{ (mol)}}\);
Allow answers such as 6.4 \( \times \) 10–2/0.064/6.3 \( \times \) 10–2/0.063 (mol).
\(m{\text{(ICl)}} = (0.0632 \times 162.35 = ){\text{ }}10.3{\text{ (g)}}\);
Allow answers in range 10.2 to 10.4 (g).
Award [3] for correct final answer.
(iii) \(\left( {\frac{{8.60}}{{10.3}} \times 100 = } \right){\text{ }}83.5\% \);
Allow answers in the range of 82.5 to 84.5%.
(iv) negative/–/minus/ < 0;
Br2 has London/dispersion/van der Waals’ forces/vdW and ICl has (London/dispersion/van der Waals’ forces/vdW and) dipole–dipole forces;
dipole–dipole forces are stronger than London/dispersion/van der Waals’/vdW forces;
Allow induced dipole-induced dipole forces for London forces.
Allow interactions instead of forces.
Do not allow ICl polar and Br2 non-polar for M1.
Name of IMF in both molecules is required for M1 and idea of dipole-dipole stronger than vdW is required for M2.
(i) \(\left( {\frac{{126.90}}{{330.71}} \times 100} \right) = 38.4\% \);
(ii) \((25.25 - 1.05) = 24.20{\text{ (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Accept 24.2 (cm3) but not 24 (cm3).
(iii) \(\left( {\frac{{24.20 \times 5.00 \times {{10}^{ - 2}}}}{{1000}}} \right) = 1.21 \times {10^{ - 3}}/0.00121{\text{ (mol)}}\);
(iv) \((0.5 \times 1.21 \times {10^{ - 3}}) = 6.05 \times {10^{ - 4}}/0.000605{\text{ (mol)}}\);
Accept alternate method e.g. (0.384/126.9 \( \times \) 0.2015) = 6.10 \( \times \) 10–4/0.000610 (mol).
(v) \((126.90 \times 6.05 \times {10^{ - 4}}) = 7.68 \times {10^{ - 2}}/0.0768{\text{ (g)}}\);
Accept alternate method e.g. (6.10 \( \times \) 10–4 \( \times \) 126.9) or (0.2015 \( \times \) 0.384) = 7.74 \( \times \) 10–2/0.00774 (g).
(vi) \(\left( {\frac{{7.68 \times {{10}^{ - 2}}}}{{0.2015}} \times 100} \right) = 38.1\% \);
Answer must be given to three significant figures.
Examiners report
This was a data based question based on quantitative chemistry. Majority of candidates were able to gain almost full marks with some candidates failing to recognise that chlorine is the limiting reagent in part (a) (ii). Some candidates calculated percentage experimental error instead of percentage yield whereas some other candidates did not pay attention to significant digits.
In part (b), explaining the difference in the boiling points of Br2 and ICl in terms of the intermolecular forces presented a challenge to many candidates. Explanations were vague or unclear and in some cases incorrect in terms of the intermolecular forces present.
In part (c), calculations of moles of iodine occasionally saw the erroneous use of Avogadro’s constant.
An acidic sample of a waste solution containing Sn2+(aq) reacted completely with K2Cr2O7 solution to form Sn4+(aq).
State the oxidation half-equation.
Deduce the overall redox equation for the reaction between acidic Sn2+(aq) and Cr2O72–(aq), using section 24 of the data booklet.
Calculate the percentage uncertainty for the mass of K2Cr2O7(s) from the given data.
The sample of K2Cr2O7(s) in (i) was dissolved in distilled water to form 0.100 dm3 solution. Calculate its molar concentration.
10.0 cm3 of the waste sample required 13.24 cm3 of the K2Cr2O7 solution. Calculate the molar concentration of Sn2+(aq) in the waste sample.
Markscheme
Sn2+(aq) → Sn4+(aq) + 2e–
Accept equilibrium sign.
Accept Sn2+(aq) – 2e– → Sn4+(aq).
[1 mark]
Cr2O72–(aq) + 14H+(aq) + 3Sn2+(aq) → 2Cr3+(aq) + 7H2O(l) + 3Sn4+(aq)
Accept equilibrium sign.
[1 mark]
«13.239 g ± 0.002 g so percentage uncertainty» 0.02 «%»
Accept answers given to greater precision, such as 0.0151%.
[1 mark]
« [K2Cr2O7] = \(\frac{{13.239{\text{ g}}}}{{294.20{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}} \times 0.100{\text{ d}}{{\text{m}}^3}}}\) =» 0.450 «mol\(\,\)dm–3»
[1 mark]
n(Sn2+) = «0.450 mol\(\,\)dm–3 x 0.01324 dm3 x \(\frac{{3\,mol}}{{1\,mol}}\) =» 0.0179 «mol»
«[Sn2+] = \(\frac{{0.0179\,mol}}{{0.0100\,mol}}\) =» 1.79 «mol\(\,\)dm–3»
Award [2] for correct final answer.
[2 marks]
Examiners report
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
Markscheme
n(Ag) = «\(\frac{{3.275{\text{ g}}}}{{107.87{\text{ g}}\,{\text{mol}}}} = \)» 0.03036 «mol»
AND
n(O) = «\(\frac{{3.760{\text{ g}} - 3.275{\text{ g}}}}{{16.00{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}} = \frac{{0.485}}{{16.00}} = \)» 0.03031 «mol»
«\(\frac{{0.03036}}{{0.03031}} \approx 1\) / ratio of Ag to O approximately 1 : 1, so»
AgO
Accept other valid methods for M1.
Award [1 max] for correct empirical formula if method not shown.
[2 marks]
temperature too low
OR
heating time too short
OR
oxide not decomposed completely
heat sample to constant mass «for three or more trials»
Accept “not heated strongly enough”.
If M1 as per markscheme, M2 can only be awarded for constant mass technique.
Accept "soot deposition" (M1) and any suitable way to reduce it (for M2).
Accept "absorbs moisture from atmosphere" (M1) and "cool in dessicator" (M2).
Award [1 max] for reference to impurity AND design improvement.
[2 marks]
Ar closer to 107/less than 108 «so more 107Ag»
OR
Ar less than the average of (107 + 109) «so more 107Ag»
Accept calculations that gives greater than 50% 107Ag.
[1 mark]
Do not accept name for the products.
Accept “Na+ + OH–” for NaOH.
Ignore coefficients in front of formula.
[3 marks]
«molten» Na2O has mobile ions/charged particles AND conducts electricity
«molten» P4O10 does not have mobile ions/charged particles AND does not conduct electricity/is poor conductor of electricity
Do not award marks without concept of mobile charges being present.
Award [1 max] if type of bonding or electrical conductivity correctly identified in each compound.
Do not accept answers based on electrons.
Award [1 max] if reference made to solution.
[2 marks]
electrons in discrete/specific/certain/different shells/energy levels
energy levels converge/get closer together at higher energies
OR
energy levels converge with distance from the nucleus
Accept appropriate diagram for M1, M2 or both.
Do not give marks for answers that refer to the lines in the spectrum.
[2 marks]
Examiners report
The structure of an organic molecule can help predict the type of reaction it can undergo.
Improvements in instrumentation have made identification of organic compounds routine.
The empirical formula of an unknown compound containing a phenyl group was found to be C4H4O. The molecular ion peak in its mass spectrum appears at m/z = 136.
The Kekulé structure of benzene suggests it should readily undergo addition reactions.
Discuss two pieces of evidence, one physical and one chemical, which suggest this is not the structure of benzene.
Formulate the ionic equation for the oxidation of propan-1-ol to the corresponding aldehyde by acidified dichromate(VI) ions. Use section 24 of the data booklet.
The aldehyde can be further oxidized to a carboxylic acid.
Outline how the experimental procedures differ for the synthesis of the aldehyde and the carboxylic acid.
Deduce the molecular formula of the compound.
Identify the bonds causing peaks A and B in the IR spectrum of the unknown compound using section 26 of the data booklet.
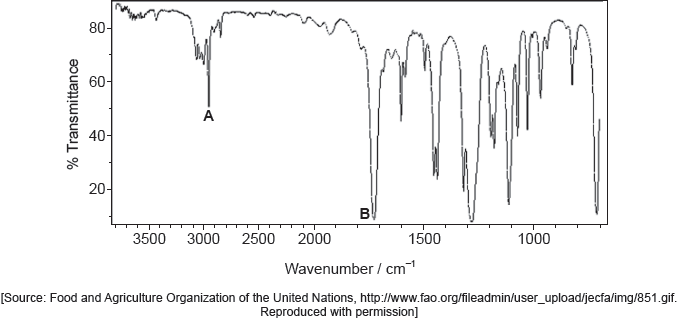
Deduce full structural formulas of two possible isomers of the unknown compound, both of which are esters.
Deduce the formula of the unknown compound based on its 1H NMR spectrum using section 27 of the data booklet.
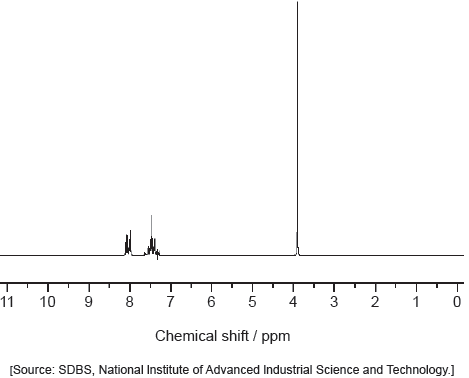
Markscheme
Physical evidence:
equal C–C bond «lengths/strengths»
OR
regular hexagon
OR
«all» C–C have bond order of 1.5
OR
«all» C–C intermediate between single and double bonds
Chemical evidence:
undergoes substitution reaction «more readily than addition»
OR
does not discolour/react with bromine water
OR
substitution forms only one isomer for 1,2-disubstitution «presence of alternate double bonds would form two isomers»
OR
more stable than expected «compared to hypothetical molecule cyclohexa-1,3,5-triene»
OR
enthalpy change of hydrogenation/combustion is less exothermic than predicted «for cyclohexa-1,3,5-triene»
M1:
Accept “all C–C–C bond angles are equal”.
[2 marks]
3CH3CH2CH2OH(l) + Cr2O72–(aq) + 8H+(aq) → 3CH3CH2CHO(aq) + 2Cr3+(aq) + 7H2O(l)
correct reactants and products
balanced equation
[2 marks]
Aldehyde:
by distillation «removed from reaction mixture as soon as formed»
Carboxylic acid:
«heat mixture under» reflux «to achieve complete oxidation to –COOH»
Accept clear diagrams or descriptions of the processes.
[2 marks]
«\(\frac{{136}}{{48 + 4 + 16}} = 2\)»
C8H8O2
[1 mark]
A: C–H «in alkanes, alkenes, arenes»
AND
B: C=O «in aldehydes, ketones, carboxylic acids and esters»
[1 mark]
Any two of:
OR C6H5COOCH3
OR CH3COOC6H5
OR HCOOCH2C6H5
Do not penalize use of Kekule structures for the phenyl group.
Accept the following structures:
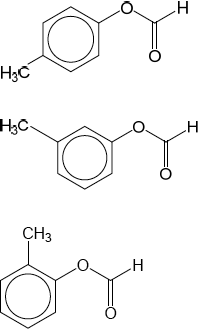
Award [1 max] for two correct aliphatic/linear esters with the molecular formula C8H8O2.
[2 marks]
C6H5COOCH3 «signal at 4 ppm (3.7 – 4.8 range in data table) due to alkyl group on ester
[1 mark]
Examiners report
The reactivity of organic compounds depends on the nature and positions of their functional groups.
The structural formulas of two organic compounds are shown below.
Deduce the type of chemical reaction and the reagents used to distinguish between these compounds.
State the observation expected for each reaction giving your reasons.
Deduce the number of signals and the ratio of areas under the signals in the 1H NMR spectra of the two compounds.
Explain, with the help of equations, the mechanism of the free-radical substitution reaction of ethane with bromine in presence of sunlight.
Markscheme
oxidation/redox AND acidified «potassium» dichromate(VI)
OR
oxidation/redox AND «acidified potassium» manganate(VII)
Accept “acidified «potassium» dichromate” OR “«acidified potassium» permanganate”.
Accept name or formula of the reagent(s).
ALTERNATIVE 1 using K2Cr2O7:
Compound A: orange to green AND secondary hydroxyl
OR
Compound A: orange to green AND hydroxyl oxidized «by chromium(VI) ions»
Compound B: no change AND tertiary hydroxyl «not oxidized by chromium(VI) ions»
Award [1] for “A: orange to green AND B: no change”.
Award [1] for “A: secondary hydroxyl AND B: tertiary hydroxyl”.
ALTERNATIVE 2 using KMnO4:
Compound A: purple to colourless AND secondary hydroxyl
OR
Compound A: purple to colourless AND hydroxyl oxidized «by manganese(VII) ions»
Compound B: no change AND tertiary hydroxyl «not oxidized by manganese(VII) ions»
Accept “alcohol” for “hydroxyl”.
Award [1] for “A: purple to colourless AND B: no change”
Award [1] for “A: secondary hydroxyl AND B: tertiary hydroxyl”.
Accept “purple to brown” for A.
Accept ratio of areas in any order.
Do not apply ECF for ratios.
Initiation:
Br2 2Br•
Propagation:
Br• + C2H6 → C2H5• + HBr
C2H5• + Br2 → C2H5Br + Br•
Termination:
Br• + Br• → Br2
OR
C2H5• + Br• → C2H5Br
OR
C2H5• + C2H5• → C4H10
Reference to UV/hν/heat not required.
Accept representation of radical without • (eg, Br, C2H5) if consistent throughout mechanism.
Accept further bromination.
Award [3 max] if initiation, propagation and termination are not stated or are incorrectly labelled for equations.
Award [3 max] if methane is used instead of ethane, and/or chlorine is used instead of bromine.
Examiners report
The Bombardier beetle sprays a mixture of hydroquinone and hydrogen peroxide to fight off predators. The reaction equation to produce the spray can be written as:
| C6H4(OH)2(aq) + H2O2(aq) | → | C6H4O2(aq) + 2H2O(l) |
| hydroquinone | quinone |
Calculate the enthalpy change, in kJ, for the spray reaction, using the data below.
\(\begin{array}{*{20}{l}} {{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{{\text{(OH)}}}_{\text{2}}}{\text{(aq)}} \to {{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{177.0 kJ}}} \\ {{\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}}}&{\Delta {H^\theta } = + {\text{189.2 kJ}}} \\ {{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}} + \frac{{\text{1}}}{{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(g)}}}&{\Delta {H^\theta } = + {\text{285.5 kJ}}} \end{array}\)
The energy released by the reaction of one mole of hydrogen peroxide with hydroquinone is used to heat 850 cm3 of water initially at 21.8°C. Determine the highest temperature reached by the water.
Specific heat capacity of water = 4.18 kJ\(\,\)kg−1\(\,\)K−1.
(If you did not obtain an answer to part (i), use a value of 200.0 kJ for the energy released, although this is not the correct answer.)
Identify the species responsible for the peak at m/z = 110 in the mass spectrum of hydroquinone.
Identify the highest m/z value in the mass spectrum of quinone.
Markscheme
ΔH = 177.0 – \(\frac{{189.2}}{2}\) –285.5 «kJ»
«ΔH =» –203.1 «kJ»
Accept other methods for correct manipulation of the three equations.
Award [2] for correct final answer.
[2 marks]
203.1 «kJ» = 0.850 «kg» x 4.18 «kJ\(\,\)kg–1\(\,\)K–1» x ΔT «K»
OR
«ΔT =» 57.2 «K»
«Tfinal = (57.2 + 21.8) °C =» 79.0 «°C» / 352.0 «K»
If 200.0 kJ was used:
200.0 «kJ» = 0.850 «kg» x 4.18 «kJ\(\,\)kg–1\(\,\)K–1» x ΔT «K»
OR
«ΔT =» 56.3 «K»
«Tfinal = (56.3 + 21.8) °C =» 78.1 «°C» / 351.1 «K»
Award [2] for correct final answer.
Units, if specified, must be consistent with the value stated.
[2 marks]
C6H4(OH)2+
Accept “molecular ion”.
Do not accept “C6H4(OH)2” (positive charge missing).
[1 mark]
«highest m/z» 108
Only accept exactly 108, not values close to this.
[1 mark]
Examiners report
This question is about carbon and chlorine compounds.
Ethane, C2H6, reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1H\(\,\)NMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
Chloroethene, C2H3Cl, can undergo polymerization. Draw a section of the polymer with three repeating units.
Markscheme
substitution AND «free-»radical
OR
substitution AND chain
Award [1] for “«free-»radical substitution” or “SR” written anywhere in the answer.
[1 mark]
Two propagation steps:
C2H6 + •Cl → C2H5• + HCl
C2H5• + Cl2 → C2H5Cl + •Cl
One termination step:
C2H5• + C2H5• → C4H10
OR
C2H5• + •Cl → C2H5Cl
OR
•Cl + •Cl → Cl2
Accept radical without • if consistent throughout.
Allow ECF from incorrect radicals produced in propagation step for M3.
[3 marks]
\({\text{C}} = \frac{{24.27}}{{12.01}}\) = 2.021 AND \({\text{H}} = \frac{{4.08}}{{1.01}}\) = 4.04 AND \({\text{Cl}} = \frac{{71.65}}{{35.45}} = 2.021\)
«hence» CH2Cl
Accept \(\frac{{24.27}}{{12.01}}\) : \(\frac{{4.08}}{{1.01}}\) : \(\frac{{71.65}}{{35.45}}.\)
Do not accept C2H4Cl2.
Award [2] for correct final answer.
[2 marks]
molecular ion peak(s) «about» m/z 100 AND «so» C2H4Cl2 «isotopes of Cl»
two signals «in 1H\(\,\)NMR spectrum» AND «so» CH3CHCl2
OR
«signals in» 3:1 ratio «in 1H\(\,\)NMR spectrum» AND «so» CH3CHCl2
OR
one doublet and one quartet «in 1H\(\,\)NMR spectrum» AND «so» CH3CHCl2
1,1-dichloroethane
Accept “peaks” for “signals”.
Allow ECF for a correct name for M3 if an incorrect chlorohydrocarbon is identified
[3 marks]
Continuation bonds must be shown.
Ignore square brackets and “n”.
Accept 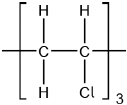 .
.
Accept other versions of the polymer, such as head to head and head to tail.
Accept condensed structure provided all C to C bonds are shown (as single).
[1 mark]
Examiners report
Alkenes are widely used in the production of polymers. The compound A, shown below, is used in the manufacture of synthetic rubber.
(i) State the name, applying IUPAC rules, of compound A.
(ii) Draw a section, showing three repeating units, of the polymer that can be formed from compound A.
(iii) Compound A is flammable. Formulate the equation for its complete combustion.
Compound B is related to compound A.
(i) State the term that is used to describe molecules that are related to each other in the same way as compound A and compound B.
(ii) Suggest a chemical test to distinguish between compound A and compound B, giving the observation you would expect for each.
Test:
Observation with A:
Observation with B:
(iii) Spectroscopic methods could also be used to distinguish between compounds A and B.
Predict one difference in the IR spectra and one difference in the 1H NMR spectra of these compounds, using sections 26 and 27 of the data booklet.
IR spectra:
1H NMR spectra:
A sample of compound A was prepared in which the 12C in the CH2 group was replaced by 13C.
(i) State the main difference between the mass spectrum of this sample and that of normal compound A.
(ii) State the structure of the nucleus and the orbital diagram of 13C in its ground state.
Draw a 1s atomic orbital and a 2p atomic orbital.
Markscheme
(i)
methylpropene
(ii)
−CH2−C(CH3)2−CH2−C(CH3)2−CH2−C(CH3)2−
Must have continuation bonds at both ends.
Accept any orientation of the monomers, which could give methyl side-chains on neighbouring atoms etc.
(iii)
C4H8 (g) + 6O2 (g) → 4CO2 (g) + 4H2O(l)
(i)
«structural/functional group» isomer«s»
(ii)
Test:
«react with» bromine/Br2 «in the dark»
OR
«react with» bromine water/Br2 (aq) «in the dark»
IR: A would absorb at 1620–1680cm−1 AND B would not
1H NMR: A would have 2 signals AND B would have 1 signal
OR
A would have a signal at 4.5–6.0 ppm AND B would not
OR
A would have a signal at 0.9–1.0 ppm AND B would not
OR
B would have a signal at 1.3–1.4 ppm AND A would not
Accept “peak” for “signal”.
Award [1 max] if students have a correct assignation of a signal, but no comparison, for both IR and NMR.
Accept “B would have a signal at 2.0 ppm” as shown in its 1H NMR spectrum.
(i)
«molecular ion» peak at «m/z =» 57, «not 56»
OR
«molecular ion» peak at one «m/z» higher
OR
will not have a «large» peak at 56
Accept a peak at m/z one greater than the 12C one for any likely fragment.
(ii)
protons: 6 AND neutrons: 7
Accept full arrows.
Accept p orbitals aligned on y− and z−axes, or diagrams correctly showing all three p-orbitals.
Do not accept p-orbitals without a node.
Examiners report
A student titrated an ethanoic acid solution, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine its concentration.
The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of acid.
Curves X and Y were obtained when a metal carbonate reacted with the same volume of ethanoic acid under two different conditions.
Using the graph, estimate the initial temperature of the solution.
Determine the maximum temperature reached in the experiment by analysing the graph.
Calculate the concentration of ethanoic acid, CH3COOH, in mol dm–3.
Determine the heat change, q, in kJ, for the neutralization reaction between ethanoic acid and sodium hydroxide.
Assume the specific heat capacities of the solutions and their densities are those of water.
Calculate the enthalpy change, ΔH, in kJ mol–1, for the reaction between ethanoic acid and sodium hydroxide.
Explain the shape of curve X in terms of the collision theory.
Suggest one possible reason for the differences between curves X and Y.
Markscheme
21.4 °C
Accept values in the range of 21.2 to 21.6 °C.
29.0 «°C»
Accept range 28.8 to 29.2 °C.
ALTERNATIVE 1
«volume CH3COOH =» 26.0 «cm3»
«[CH3COOH] = 0.995 mol dm–3 \( \times \frac{{50.0\,{\text{cm3}}}}{{26.0\,{\text{cm3}}}} = \)» 1.91 «mol dm−3»
ALTERNATIVE 2
«n(NaOH) =0.995 mol dm–3 x 0.0500 dm3 =» 0.04975 «mol»
«[CH3COOH] = \(\frac{{0.04975}}{{0.0260}}\) dm3 =» 1.91 «mol dm–3»
Accept values of volume in range 25.5 to 26.5 cm3.
Award [2] for correct final answer.
«total volume = 50.0 + 26.0 =» 76.0 cm3 AND «temperature change 29.0 – 21.4 =» 7.6 «°C»
«q = 0.0760 kg x 4.18 kJ kg–1 K–1 x 7.6 K =» 2.4 «kJ»
Award [2] for correct final answer.
«n(NaOH) = 0.995 mol dm–3 x 0.0500 dm3 =» 0.04975 «mol»
OR
«n(CH3COOH) = 1.91 mol dm–3 x 0.0260 dm3 =» 0.04966 «mol»
«ΔH = \( - \frac{{2.4\,{\text{kJ}}}}{{0.04975\,{\text{mol}}}} = \)» –48 / –49 «kJ mol–1»
Award [2] for correct final answer.
Negative sign is required for M2.
«initially steep because» greatest concentration/number of particles at start
OR
«slope decreases because» concentration/number of particles decreases
volume produced per unit of time depends on frequency of collisions
OR
rate depends on frequency of collisions
mass/amount/concentration of metal carbonate more in X
OR
concentration/amount of CH3COOH more in X
Examiners report
Sodium thiosulfate solution reacts with dilute hydrochloric acid to form a precipitate of sulfur at room temperature.
Na2S2O3 (aq) + 2HCl (aq) → S (s) + SO2 (g) + 2NaCl (aq) + X
Identify the formula and state symbol of X.
Suggest why the experiment should be carried out in a fume hood or in a well-ventilated laboratory.
The precipitate of sulfur makes the mixture cloudy, so a mark underneath the reaction mixture becomes invisible with time.
10.0 cm3 of 2.00 mol dm-3 hydrochloric acid was added to a 50.0 cm3 solution of sodium thiosulfate at temperature, T1. Students measured the time taken for the mark to be no longer visible to the naked eye. The experiment was repeated at different concentrations of sodium thiosulfate.
Show that the hydrochloric acid added to the flask in experiment 1 is in excess.
Draw the best fit line of \(\frac{1}{{\rm{t}}}\) against concentration of sodium thiosulfate on the axes provided.
A student decided to carry out another experiment using 0.075 mol dm-3 solution of sodium thiosulfate under the same conditions. Determine the time taken for the mark to be no longer visible.
An additional experiment was carried out at a higher temperature, T2.
(i) On the same axes, sketch Maxwell–Boltzmann energy distribution curves at the two temperatures T1 and T2, where T2 > T1.
(ii) Explain why a higher temperature causes the rate of reaction to increase.
Suggest one reason why the values of rates of reactions obtained at higher temperatures may be less accurate.
Markscheme
H2O AND (l)
Do not accept H2O (aq).
SO2 (g) is an irritant/causes breathing problems
OR
SO2 (g) is poisonous/toxic
Accept SO2 (g) is acidic, but do not accept “causes acid rain”.
Accept SO2 (g) is harmful.
Accept SO2 (g) has a foul/pungent smell.
n(HCl) = «\(\frac{{10.0}}{{1000}}\)dm3 × 2.00 mol dm-3 =» 0.0200 / 2.00 × 10-2«mol»
AND
n(Na2S2O3) = «\(\frac{{50}}{{1000}}\)dm3 × 0.150 mol × dm-3 =» 0.00750 / 7.50 × 10-3 «mol»
0.0200 «mol» > 0.0150 «mol»
OR
2.00 × 10-2«mol» > 2 × 7.50 × 10-3 «mol»
OR
\(\frac{1}{2}\) × 2.00 × 10-2 «mol» > 7.50 × 10-3 «mol»
Accept answers based on volume of solutions required for complete reaction.
Award [2] for second marking point.
Do not award M2 unless factor of 2 (or half) is used.
five points plotted correctly
best fit line drawn with ruler, going through the origin
22.5 × 10-3 «s-1»
«Time = \(\frac{1}{{22.5 \times {{10}^{ - 3}}}}\) =» 44.4 «s»
Award [2] for correct final answer.
Accept value based on candidate’s graph.
Award M2 as ECF from M1.
Award [1 max] for methods involving taking mean of appropriate pairs of \(\frac{1}{{\rm{t}}}\) values.
Award [0] for taking mean of pairs of time values.
Award [2] for answers between 42.4 and 46.4 «s».
(i)
correctly labelled axes
peak of T2 curve lower AND to the right of T1 curve
Accept “probability «density» / number of particles / N / fraction” on y-axis.
Accept “kinetic E/KE/EK” but not just “Energy/E” on x-axis.
(ii)
greater proportion of molecules have E ≥ Ea or E > Ea
OR
greater area under curve to the right of the Ea
greater frequency of collisions «between molecules»
OR
more collisions per unit time/second
Accept more molecules have energy greater than Ea.
Do not accept just “particles have greater kinetic energy”.
Accept “rate/chance/probability/likelihood/” instead of “frequency”.
Accept suitably shaded/annotated diagram.
Do not accept just “more collisions”.
shorter reaction time so larger «%» error in timing/seeing when mark disappears
Accept cooling of reaction mixture during course of reaction.